Marketing Hotline:
(+86)0532-88988868
(+86)0532-88988868
In developed countries, the vast majority of water plants use special water treatment salts as consumables for sodium hypochlorite generators. Take a leading European industry brand as an example: when providing equipment, they also supply specialized electrolytic salts, which are standard for the deployment of drinking water disinfection equipment in developed countries. In China, online sodium hypochlorite generation systems still widely use ordinary non-iodized edible salt purchased by the users as consumables.
However, the national standard for edible salt GB/T5461 does not specify its suitability for water treatment applications; the trace amounts of bromine in edible salt can produce toxic carcinogens under electrolysis, affecting the safety of tap water; the anti-caking agents in edible salt can generate by-products such as trivalent iron during electrolysis, impacting the stable operation and lifespan of equipment and increasing maintenance costs. In contrast, salts intended for water treatment strictly control a range of trace elements related to electrolysis applications. The practice of using edible salt in place of water treatment salt currently falls into a regulatory grey area at the national level.
Edible salt cannot replace salt used for water treatment
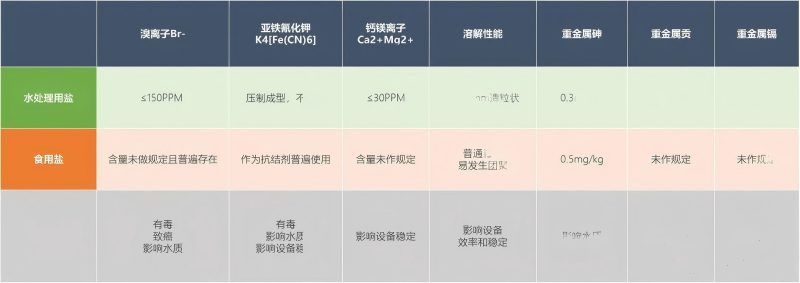
The impact of the above indicators on the sanitary quality of drinking water:
1. Bromide ions (Br-) form bromate through electrolysis, and bromates are recognized by international organizations as potential carcinogens! When the bromide ion content in disinfectant salt is ≤150 PPM, the bromate content derived from the disinfectant salt can be controlled at 0.001 PPM, far below the national standard GB5749 for bromate content in drinking water, which is 0.01 PPM.
2. Table salt commonly uses potassium ferrocyanide K4[Fe(CN)6] as an anti-caking agent. Under electrolysis, potassium ferrocyanide can produce the toxic substance potassium ferricyanide, which can cause kidney damage. Additionally, it can further produce hydrogen cyanide (HCN) under ultraviolet light, sunlight, heating, or acidic conditions, which is toxic. When burned at high temperatures, it completely decomposes into highly toxic potassium cyanide. The national standard GB5749 for drinking water hygiene sets the limit for iron ions (Fe) at ≤0.3 PPM. Introducing potassium ferrocyanide can also increase the iron ion content in water; moreover, under electrolysis, it can produce ferrous ferrocyanide precipitate, affecting water quality and color.
3. According to the national standard GB5749 for living water hygiene, the limits for heavy metals are as follows: Lead 0.01 mg/L, Arsenic 0.01 mg/L, Mercury 0.001 mg/L, Cadmium 0.005 mg/L, Barium N/A. The naturally 'low' heavy metal content in salts used for water treatment will provide drinking water with relatively ideal heavy metal levels.

The impact of the above indicators on the operation of sodium hypochlorite generation equipment:
1. The Fe3O4 produced by the electrolysis of potassium ferrocyanide poses a risk of piercing or blocking the diaphragm in generators with a diaphragm; for generators without a diaphragm, it increases energy consumption and carries the risk of producing unnecessary by-products.
2. The presence of calcium and magnesium ions in salt not only increases the electricity consumption during electrolysis in sodium hypochlorite generators but also causes scaling, affecting the stable operation of the generator. Only when the generator operates stably can the disinfection process in water purification companies be reliable, ensuring the safety of water for countless households.
3. Ordinary granular table salt will form a relatively stable salt layer at the bottom of the water, making it difficult to fully dissolve. It may also easily lead to a supersaturated solution, where some crystals remain undissolved and suspended in the solution, which can then be drawn into the electrolytic equipment. This portion of non-liquid crystals cannot be electrolyzed, causing waste, and in severe cases, it can lead to poor operation of the generator. On the other hand, water treatment salt in granulated form can avoid caking issues during transportation and storage without the addition of potassium ferrocyanide (an anti-caking agent); at the same time, the large gaps between the granules facilitate water penetration, helping the salt dissolve evenly and quickly, aiding in the formation of a stable saturated solution, and maintaining the smooth operation of the equipment.
Advantages of Using Salt for Water Treatment

Compliant with policies and industry standards
The industry standard for salt used in water treatment has been formulated and issued by relevant institutions designated by the Ministry of Industry and Information Technology. This standard serves to regulate the use of salt in the water treatment industry.
Reduce by-products and meet water quality improvement requirements
Compared to table salt, salt used for water treatment meets the highest technical standards in terms of bromide and heavy metal content, preventing situations in water plant production where non-compliant specifications could lead to 'intentional contamination.' It meets the water quality standard upgrade requirements set by local governments, especially in densely populated cities.
Conducive to equipment maintenance and reduces maintenance costs
Salt used for water treatment has strict specifications for calcium and magnesium ion content, potassium ferrocyanide content, and solubility compared to table salt, which helps ensure stable operation of equipment and reduces daily maintenance costs.


